

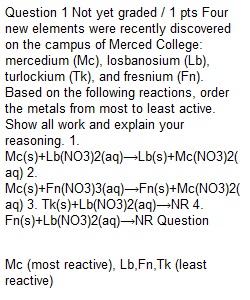
Q Question 1 Not yet graded / 1 pts Four new elements were recently discovered on the campus of Merced College: mercedium (Mc), losbanosium (Lb), turlockium (Tk), and fresnium (Fn). Based on the following reactions, order the metals from most to least active. Show all work and explain your reasoning. 1. Mc(s)+Lb(NO3)2(aq)?Lb(s)+Mc(NO3)2(aq) 2. Mc(s)+Fn(NO3)3(aq)?Fn(s)+Mc(NO3)2(aq) 3. Tk(s)+Lb(NO3)2(aq)?NR 4. Fn(s)+Lb(NO3)2(aq)?NR Question 2 Not yet graded / 1 pts The extra terrestrials who banished the Imperial unit system also got ahold of our periodic table. They reorganized it as shown below, but kept same element names and symbols we are familiar with. They also kept the s, p, d and f blocks in place (but adjusted the number of orbitals in each subshell). Use the ET periodic table below to answer the following question. Potassium has an ionization energy (IE) trend as follows: IE3 >> IE2 > IE1. Explain in terms of electron configurations. Question 3 Not yet graded / 1 pts Write the total and net ionic equations for the following reaction. Make an atomic level sketch (in a beaker) of the species present after the reaction is complete. Fn(NO3)3(aq)+LbSO4(aq)?Fn2(SO4)3(s)+Lb(NO3)2(aq) Question 4 Not yet graded / 1 pts A student makes the following claim. "Nitric acid and sodium hydroxide completely react (no reactants - of either type) and a non-conducting solution is formed. " Do you support or refute that statement? Explain. Question 5 1 / 1 pts Use the meat-veggie periodic table below to write the chemical formnula for: triblueberrogen hexaapplide Do not use any spaces or special formatting. Enter Na2CO3 as Na2CO3. ________________________________________ Food Land Parent Ions applate ion, AO43? choysumate ion, CsO42? pumpkinate ion, PuO3? broccate ion, BO42? cucumbate ion, CmO3? zuccinate ion, ZO3? cabbate ion, CO42? pearate ion, PO43?
View Related Questions